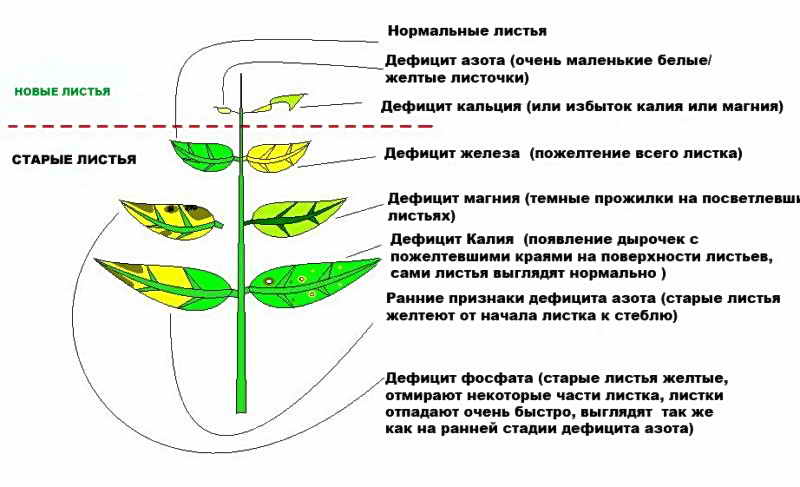
Just like humans and animals, plants have vital nutrients that they obtain from soil, water and air. The composition of the soil directly affects the health of the plant, because it is in the soil that the main trace elements are found: iron, potassium, calcium, phosphorus, manganese and many others. If some element is missing, the plant gets sick and may even die. However, an overabundance of minerals is no less dangerous.
How to find out which element in the soil is insufficient or, conversely, too much? Soil analysis is carried out by special research laboratories, and all large crop farms resort to their services. But what can simple gardeners and lovers of home flowers do, how can you independently diagnose a lack of nutrients? It's simple: if the soil lacks iron, phosphorus, magnesium and any other substance, the plant itself will tell you about this, because the health and appearance of a green pet depends, among other things, on the amount of mineral elements in the soil. In the table below, you can see a summary of the symptoms and causes of the disease.

Let us consider in more detail the symptoms of a lack and an overabundance of certain substances.
Features of the nutrition process
Being the main source of energy, without which all life processes are extinguished, food is necessary for every organism. Consequently, nutrition is not just important, but one of the basic conditions for the high-quality growth of a plant, and they get food, using all the aboveground parts and the root system. Through the roots, they extract water and the necessary mineral salts from the soil, replenishing the necessary supply of substances, carrying out soil or mineral nutrition of plants.
An essential role in this process is assigned to root hairs, therefore such nutrition is also called root. With the help of these filamentous hairs, the plant draws water solutions of various chemical elements from the ground.
They work on the principle of a pump and are located at the root in the suction zone. Salt solutions entering the hair tissue move to the conducting cells - tracheids and blood vessels. Through them, substances enter the wired zones of the root, then along the stems they spread to all above-ground parts.
Absorption
The main source of trace elements for plants is their nutrient medium, i.e. nutrient solutions or soils. The connection of trace elements with soil components is one of the most important factors determining their bioavailability. In general, plants easily absorb forms of trace elements dissolved in soil solutions, both ionic and chelates and complexes. Its main features can be summarized as follows:
- Absorption usually occurs at very low levels in solutions.
- Absorption is highly dependent on the concentration in the solution, especially at a low concentration.
- Its rate strongly depends on the concentration of H + and other ions.
- The intensity varies depending on the type of plant and the stage of development.
- The absorption processes are sensitive to such properties of the soil environment as temperature, aeration, redox potential.
- Absorption can be selective for certain ions.
- The accumulation of some ions can occur in the direction opposite to the gradient of their concentrations in the soil.
- In the circulation of the element between the roots and the external environment, mycorrhiza plays an important role.
Such generalized schemes of the processes acting during the absorption of microelements by a plant are usually fully valid for one or several elements, but more often they represent a kind of approximation of the processes operating in the natural plant - soil system. The main route of entry of trace elements into the plant is absorption by the roots, however, the ability of other tissues to easily absorb some nutrient components has been noted.
Absorption by roots
The uptake of trace elements by the roots can be passive (non-metabolic) and active (metabolic).
Passive absorption occurs by diffusion of ions from the external solution into the root endoderm. Active absorption requires the expenditure of energy of metabolic processes, and it is directed against chemical gradients. A number of data confirm the assumption that at normal concentrations in the soil solution, the uptake of trace elements by plant roots is controlled by metabolic processes within the roots themselves.
There is a lot of evidence that the root system of plants is very active in transferring trace elements associated with various soil components into a mobile state. The most accessible to plants are those microelements that are adsorbed on clay minerals (especially montmorillonite and illite), while those fixed on oxides and bound by microorganisms are less available. The drop in the concentration of microelements in the solution near the root surface, found in a number of cases, reflects a higher rate of absorption by the roots in comparison with their diffusion and convective transfer in the soil. Several processes are involved in the absorption of trace elements by the roots:
- cation exchange with the root system;
- intracellular transport by chelating agents or other carriers;
- action of the rhizosphere.
Ions and other substances released by roots into the environment affect the absorption of nutrients by the latter. Apparently, these processes are of great importance for the oxidation state of cations. Changes in the pH of the surrounding roots can play a particularly important role in the availability of certain trace elements.
The ability of different plants to absorb trace elements is highly variable. However, when considered as a whole, the bioaccumulation potential of trace elements shows some general trends. Elements such as Cd, B, Br, Cs, Rb are absorbed extremely easily, while Ba, Ti, Zr, Sc, Bi, Ga, and to some extent Fe and Se are only poorly available to plants (Figure 1).
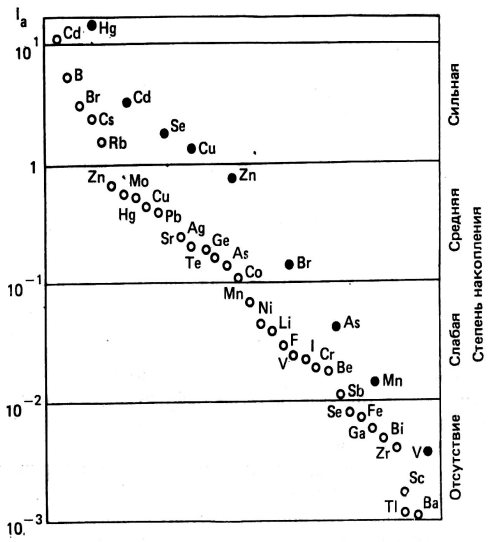

Light circles - green plants; dark circles are mushrooms. Figure 1 - Bioaccumulation of trace elements by plants relative to the soil. The accumulation index lа was calculated as the ratio of the contents of trace elements in the plant to their concentrations in the soil.
Fungi are non-photosynthetic plants with a significantly different feeding mechanism; they have a specific affinity for certain trace elements. Fungi can accumulate Hg, as well as Cd, Se, Cu, Zn and other elements to high concentrations (Figure 1).
Absorption by leaves
The bioavailability of micronutrients from air sources through the leaves (foliar uptake) can have a significant impact on plant contamination. This is also of practical importance for foliar feeding, especially with elements such as Fe, Mn, Zn and Cu. Foliar absorption of radionuclides that enter the atmosphere during nuclear weapons tests and the operation of atomic energy enterprises is now particularly alarming.
Foliar uptake is believed to have two phases - non-metabolic penetration through the cuticle, which is generally regarded as the main route of entry, and metabolic processes that account for the accumulation of elements opposite to concentration gradients. The second group of processes is responsible for the transfer of ions across plasma membranes and into the protoplasm of cells.
Trace elements absorbed by leaves can be transferred to other plant tissues, including roots, where excess amounts of some elements can be stored. The speed of movement of trace elements in tissues varies greatly depending on the organ of the plant, its age and the nature of the element. The results shown in Figure 2 show that Cd, Zn and Pb absorbed by the aboveground mass of plants (experimental plant - fire), apparently, cannot quickly move to the roots, while Cu is very mobile.
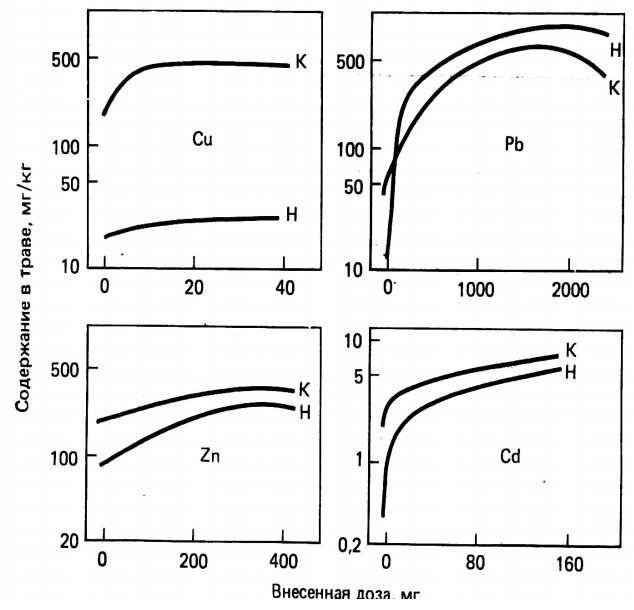

Figure 2 - Distribution of heavy metals coming from atmospheric sources between the ground mass of a plant (H) and roots (K)
Some of the trace elements captured by the leaves can be washed out with rainwater. Differences in the leaching efficiency of different trace elements can be compared with their functions or metabolic links. For example, the readily occurring removal of Pb by flushing suggests that this element is present mainly as a sediment on the leaf surface. In contrast, the small proportion of Cu, Zn and Cd that can be washed away indicates a significant penetration of these metals into the leaves. Substantial uptake of foliar-applied Zn, Fe, Cd and Hg has been reported. Washing of elements from leaves by acid rain can involve cation exchange processes, in which the H + ion of rainwater replaces microcations held in a bound position on the cuticle of the leaves.
Elements of mineral nutrition of plants
So, substances obtained from the soil serve as food for the representatives of the plant kingdom. Plant nutrition, whether mineral or soil, is a unity of different processes: from absorption and advancement to assimilation of elements found in the soil in the form of mineral salts.


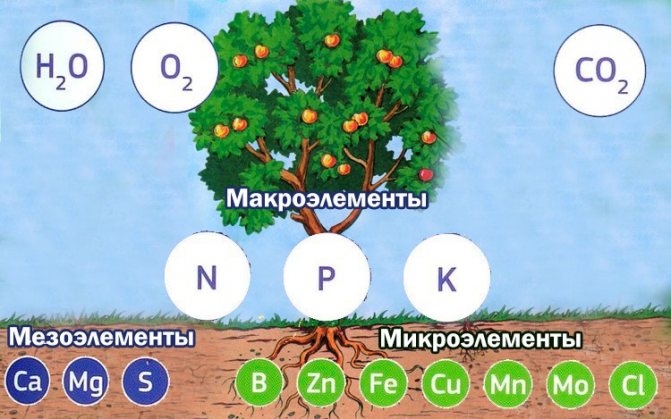
Studies of the ash left over from plants have shown how many chemical elements remain in it and their amount in different parts and different representatives of the flora is not the same. This is evidence that chemical elements are absorbed and accumulate in plants. Similar experiments led to the following conclusions: elements found in all plants - phosphorus, calcium, potassium, sulfur, iron, magnesium, as well as trace elements represented by zinc, copper, boron, manganese, etc. are recognized as vital.
Despite the different amounts of these substances, they are present in any plant, and the replacement of one element with another is impossible under any conditions. The level of the presence of minerals in the soil is very important, since the yield of agricultural crops and the decorativeness of flowering ones depend on it. In different soils, the degree of saturation of the soil with the necessary substances is also different. For example, in the temperate latitudes of Russia, there is a significant shortage of nitrogen and phosphorus, sometimes potassium, so it is mandatory to apply fertilizers - nitrogen and potassium-phosphorus. Each element has its own role in the life of the plant organism.
Proper plant nutrition (mineral) stimulates quality development, which is carried out only when all the necessary substances in the right amount are present in the soil. If there is a shortage or excess of some of them, the plants react by changing the color of the foliage. Therefore, one of the important conditions for agricultural crops is the developed norms for the introduction of fertilizers and fertilizers.Note that underfeeding is better for many plants than overfeeding. For example, for all berry horticultural crops and their wild-growing forms, it is precisely the excess of nutrition that is destructive. We will learn how different substances interact with plant tissues, and what each of them affects.
How soil nutrition is carried out
Root hairs absorb soil water.
Fig. 2. Root hairs.
Then the water moves to the vessels of the xylem, through which it rises to the aboveground organs.
Absorption is due to osmosis. This physical phenomenon denotes the movement of water to an area of higher concentration of solutes. Of course, the mineral content in the root is higher than in the soil, and therefore water is absorbed by the root.
Fig. 3. Scheme of water movement in the root.
Rhizome, tuber and old roots do not absorb water. Absorption occurs only in growing roots, up to 5 cm from the tops.
Nitrogen
One of the most essential elements for plant growth is nitrogen. It is present in proteins and amino acids. Nitrogen deficiency manifests itself in a change in the color of the leaves: at first, the leaf becomes smaller and reddens. A significant deficiency causes an unhealthy yellow-green or bronze-red patina. Older leaves on the bottom of the shoots are affected first, then along the entire stem. With continued deficiency, branch growth and fruit set stops.


Excessive fertilization with nitrogen compounds leads to an increased nitrogen content in the soil. At the same time, a rapid growth of shoots and an intensive build-up of green mass are observed, which prevents the plant from laying flower buds. As a result, the productivity of the plant is markedly reduced. This is why balanced mineral soil nutrition of plants is so important.
Micronutrient deficiency
Most often, the plant experiences a deficiency of certain microelements in the case when the composition of the soil is not balanced. Too high or, conversely, low acidity, excessive content of sand, peat, lime, black soil - all this leads to a lack of any mineral component. The content of trace elements is also influenced by weather conditions, especially excessively low temperatures.
Usually, the symptoms characteristic of micronutrient deficiencies are pronounced and do not overlap with each other, so it is quite easy to identify the lack of nutrients, especially for an experienced gardener.
[!] Do not confuse the external manifestations, characteristic of a lack of minerals, with the manifestations that occur in the case of plant damage by viral or fungal diseases, as well as various types of insect pests.
Iron - an element vital for a plant, participating in the process of photosynthesis and accumulating mainly in the leaves.
Lack of iron in the soil, and hence in the nutrition of the plant, is one of the most common diseases called chlorosis. And, although chlorosis is a symptom that is also characteristic of a deficiency of magnesium, nitrogen and many other elements, iron deficiency is the first and main cause of chlorosis. Signs of iron chlorosis are yellowing or whitening of the interveinal space of the leaf plate, while the color of the veins themselves does not change. First of all, the upper (young) leaves are affected. The growth and development of the plant does not stop, but the newly emerging shoots have an unhealthy chlorotic color. Iron deficiency most often occurs in acidic soils.
Iron deficiency is treated with special preparations containing iron chelate: Ferrovit, Mikom-Reak Iron Chelate, Micro-Fe. Iron chelate can also be made by yourself by mixing 4g. ferrous sulfate from 1 liter. water and adding 2.5 g to the solution. citric acid. One of the most effective folk remedies for iron deficiency is to stick a few old rusty nails into the soil.
[!] How do you know that the iron content in the soil has returned to normal? Young, growing leaves are normal green in color.


Magnesium. About 20% of this substance is contained in the chlorophyll of the plant. This means that magnesium is essential for proper photosynthesis. In addition, the mineral is involved in redox processes
When there is not enough magnesium in the soil, chlorosis also occurs on the leaves of the plant. But, unlike the signs of iron chlorosis, the lower, older leaves first of all suffer. The color of the leaf plate between the veins changes to reddish, yellowish. Spots appear throughout the leaf, indicating tissue dying. The veins themselves do not change their color, and the general color of the leaves resembles a herringbone pattern. Often, with a lack of magnesium, you can see deformation of the sheet: curling and wrinkling of the edges.
To eliminate the lack of magnesium, special fertilizers are used that contain a large amount of the necessary substance - dolomite flour, potassium magnesium, magnesium sulfate. Wood ash and ash make up the magnesium deficiency well.


Copper important for the correct protein and carbohydrate processes in the plant cell and, accordingly, the development of the plant.
Excessive content of peat (humus) and sand in the soil mixture often leads to a copper deficiency. Popularly, this disease is called the white plague or white-mouthed. Citrus houseplants, tomatoes, and cereals are especially sensitive to the lack of copper. The following signs will help to identify the lack of copper in the soil: general lethargy of leaves and stems, especially the upper ones, delay and arrest of the growth of new shoots, the death of the apical bud, white spots at the tip of the leaf or throughout the leaf plate. In cereals, leaf twisting into a spiral is sometimes observed.
For the treatment of copper deficiency, copper-containing fertilizers are used: superphosphate with copper, copper sulfate, pyrite cinders.


Zinc has a great influence on the rate of redox processes, as well as on the synthesis of nitrogen, carbohydrates and starches.
Zinc deficiency usually manifests itself in acidic marsh or sandy soils. Symptoms of zinc deficiency are usually localized on the leaves of the plant. This is a general yellowing of the leaf or the appearance of individual spots, often spots become more saturated, bronze color. Subsequently, the tissue dies off in such areas. First of all, symptoms appear on the old (lower) leaves of the plant, gradually rising higher and higher. In some cases, spots may appear on the stems as well. Newly appearing leaves are abnormally small and covered with yellow specks. Sometimes you can observe the upward curling of the sheet.
In case of zinc deficiency, zinc-containing complex fertilizers or zinc sulfate are used.


Bor. With the help of this element, the plant fights viral and bacterial diseases. In addition, boron is actively involved in the growth and development of new shoots, buds, and fruits.
Swampy, calcareous and acidic soils very often lead to boric starvation of the plant. Various types of beets and cabbage suffer especially from boron deficiency. Boron deficiency symptoms appear, first of all, on young shoots and upper leaves of the plant. The color of the leaves changes to light green, the leaf plate is twisted into a horizontal tube. The veins of the leaf turn dark, even black, and break when bent. The upper shoots are especially affected, up to death, the growing point is affected, as a result of which the plant develops with the help of lateral processes. The formation of flowers and ovaries slows down or completely stops, the flowers and fruits that have already appeared are crumbled.
Boric acid will help to compensate for the lack of boron.
[!] It is necessary to use boric acid with the utmost care: even a small overdose will lead to the death of the plant.


Molybdenum. Molybdenum is essential for photosynthesis, synthesis of vitamins, nitrogen and phosphorus metabolism, in addition, the mineral is a component of many plant enzymes.
If a large number of brown or brown specks appear on the old (lower) leaves of the plant, and the veins remain of a normal green color, the plant may lack molybdenum. In this case, the surface of the leaf is deformed, swelling, and the edges of the leaves curl. New young leaves do not change color at first, but over time, mottling appears on them. The manifestation of molybdenum deficiency is called "Viptail Disease"
Molybdenum deficiency can be compensated for with fertilizers such as ammonium molybdate and ammonium molybdate.


Manganese necessary for the synthesis of ascorbic acid and sugars. In addition, the element increases the content of chlorophyll in the leaves, increases the plant's resistance to unfavorable factors, and improves fruiting.
Manganese deficiency is determined by the pronounced chlorous color of the leaves: the central and lateral veins remain a rich green color, and the interveinal tissue becomes lighter (becomes light green or yellowish). Unlike iron chlorosis, the pattern is not so pronounced, and the yellowness is not so bright. At first, symptoms can be seen at the base of the upper leaves. Over time, as the leaves age, the chlorotic pattern diffuses, and stripes appear on the leaf blade along the central vein.
For the treatment of manganese deficiency, manganese sulfate or complex fertilizers containing manganese are used. From folk remedies, you can use a weak solution of potassium permanganate or diluted manure.


Nitrogen - one of the most important elements for a plant. There are two forms of nitrogen, one of which is necessary for oxidative processes in the plant, and the other for reductive ones. Nitrogen helps to maintain the required water balance and stimulates plant growth and development.
Most often, a lack of nitrogen in the soil occurs in early spring, due to low soil temperatures, which prevent the formation of minerals. Nitrogen deficiency is most pronounced at the stage of early plant development: thin and sluggish shoots, small leaves and inflorescences, low branching. In general, the plant does not develop well. In addition, the lack of nitrogen can be indicated by a change in leaf color, in particular, the color of the veins, both central and lateral. With nitrogen starvation, the veins first turn yellow, and then the leaf veins turn yellow. Also, the color of the veins and leaves can become reddish, brown or light green. Symptoms appear primarily on older leaves, eventually affecting the entire plant.
Lack of nitrogen can be replenished with fertilizers containing nitrate nitrogen (potassium, ammonium, sodium and other nitrates) or ammonium nitrogen (ammophos, ammonium sulfate, urea). A high nitrogen content is present in natural organic fertilizers.
[!] In the second half of the year, nitrogen fertilizers should be excluded, as they can impede the transition of the plant from dormancy and preparation for wintering.


Phosphorus. This trace element is especially important during flowering and fruit formation, as it stimulates plant development, including fruiting. Phosphorus is also necessary for proper wintering, so the best time to apply fluoride fertilizers is the second half of summer.
Signs of phosphorus deficiency are difficult to confuse with any other symptoms: leaves and shoots are stained bluish, the glossiness of the leaf surface is lost. In especially advanced cases, the color may even be purple, purple or bronze. On the lower leaves, areas of dead tissue appear, then the leaf completely dries out and falls off. Fallen leaves are dark, almost black.At the same time, young shoots continue to develop, but they look weakened and depressed. In general, the lack of phosphorus affects the general development of the plant - the formation of inflorescences and fruits slows down, and the yield decreases.
Treatment of phosphorus deficiency is carried out with the help of phosphorus fertilizers: phosphate flour, potassium phosphate, superphosphate. Poultry manure contains a large amount of phosphorus. Ready-made phosphorus fertilizers dissolve in water for a long time, so they must be applied in advance.


Potassium - one of the main elements of the mineral nutrition of the plant. Its role is enormous: maintaining water balance, enhancing plant immunity, enhancing resistance to stress, and much more.
An insufficient amount of potassium leads to a marginal burn of the leaf (deformation of the leaf edge, accompanied by drying). Brown spots appear on the leaf plate, the veins look as if pressed into the leaf. Symptoms appear primarily on older leaves. Often, a lack of potassium leads to active leaf fall during the flowering period. The stems and shoots droop, the development of the plant slows down: the appearance of new buds and shoots, the setting of fruits, is suspended. Even if new shoots grow, their shape is underdeveloped and ugly.
Such supplements as potassium chloride, potassium magnesium, potassium sulfate, wood ash help to fill the lack of potassium.


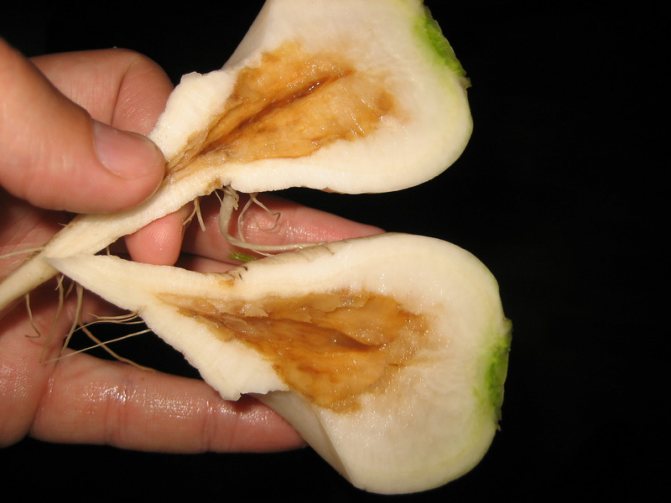
Calcium important for the proper functioning of plant cells, protein and carbohydrate metabolism. The root system is the first to suffer from a lack of calcium.
Signs of calcium deficiency are manifested, first of all, on young leaves and shoots: brown spotting, curvature, twisting. Later, already formed and newly emerging shoots die off. Lack of calcium leads to impaired assimilation of other minerals, therefore, signs of potassium, nitrogen or magnesium starvation may appear on the plant.
[!] It should be noted that indoor plants rarely suffer from calcium deficiency, since tap water contains quite a lot of salts of this substance.
Lime fertilizers help to increase the amount of calcium in the soil: chalk, dolomite limestone, dolomite flour, slaked lime and many others.


Phosphorus
This element is no less important in plant life. It is a constituent part of nucleic acids, the combination of which with proteins form nucleoproteins that are part of the cell nucleus. Phosphorus is concentrated in plant tissues, flowers and seeds. In many ways, the ability of trees to withstand natural disasters depends on the presence of phosphorus. He is responsible for frost resistance and comfortable wintering. Deficiency of the element manifests itself in a slowdown in cell division, cessation of plant growth and development of the root system, the foliage acquires a lilac-red hue. The aggravation of the situation threatens the plant with death.
Moving
The transfer of ions in plant tissues and organs involves several processes:
- movement in the xylem;
- movement in the phloem;
- storage, accumulation and transition to a stationary state.
Chelating ligands are most important for the transport of cations in plants. However, many other factors also affect the mobility of metals in plant tissues: pH, redox conditions, competition between cations, hydrolysis, polymerization, and the formation of insoluble salts (for example, phosphates, oxalates, etc.).
Tiffin provides a detailed review of the mechanisms involved in the transfer of micro nutrient components in plants. In general, the distant transfer of trace elements in higher plants depends on the activity of vascular tissues (xylem and phloem) and is partially related to the intensity of transpiration. The chemical forms of trace elements in phloem excretions are different for different elements.It is reported, for example, that Zn is almost entirely bound to organic substances, while Mn is only partly bound into complexes.
The distribution and accumulation of trace elements varies markedly for different elements, plant species and growth seasons. In the phase of intense rbeta of spring barley, the contents of Fe and Mn are relatively low, while the contents of Cu and Zn are very high. While the first two elements accumulate mainly in old leaves and leaf sheaths, Cu and Zn appear to be more uniformly distributed throughout the plant. The differentiated distribution of trace elements between different parts of the pine is clearly seen from Table 1. The accumulation and immobilization of trace elements in the roots is a relatively common phenomenon, especially if they are sufficiently supplied.
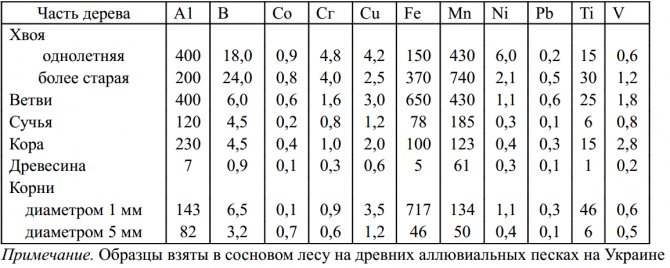

Table 1 - Variations in the content of trace elements in pines (mg / kg dry weight)
Potassium
The mineral substances for plant nutrition include potassium. It is necessary in the greatest quantities, since it stimulates the process of absorption, biosynthesis and transport of vital elements to all parts of the plant.


Normal supply of potassium increases the resistance of the plant organism, stimulates defense mechanisms, drought and cold resistance. Flowering and fruit formation with a sufficient supply of potassium is more efficient: flowers and fruits are much larger and brighter in color.
With a lack of an element, growth slows down significantly, and a strong deficiency leads to thinning and fragility of the stems, a change in the color of the leaves to purple-bronze. Then the leaves dry and collapse.
Bioavailability
Figure 3 illustrates the linear response of the absorption of trace elements by many plant species to an increase in their concentrations in nutrient and soil solutions. This response confirms the conclusion that the most reliable methods for establishing the availability of trace elements in soils are methods based on the concentrations of elements in soil solutions, and not on the determination of the stock of soluble and / or exchangeable trace elements.
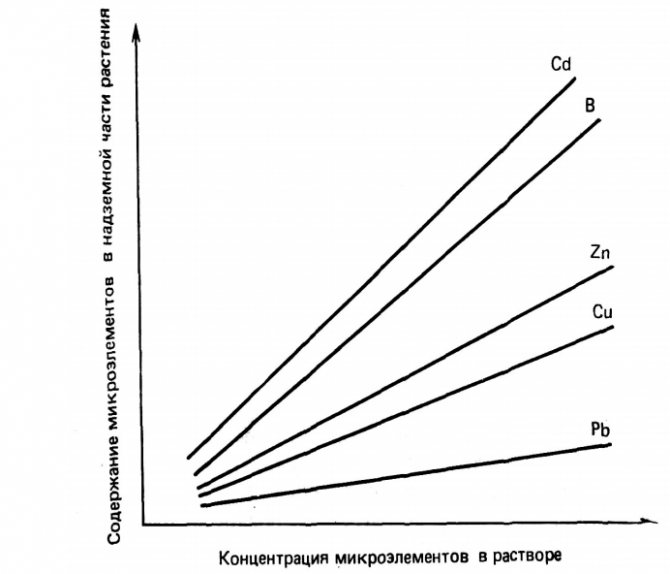

Figure 3 - Absorption of trace elements by plants depending on their concentration in nutrient solutions
When determining the bioavailability of trace elements, the specific properties of plants are very important. They vary quite a bit depending on soil conditions and plant conditions. The ability of different plant species to absorb some trace elements from the same soil environment is illustrated in Table 2. From the data presented, it follows that to obtain an effective estimate of the stock of biologically available trace elements, it is necessary to jointly apply methods based on soil tests and plant analysis data.
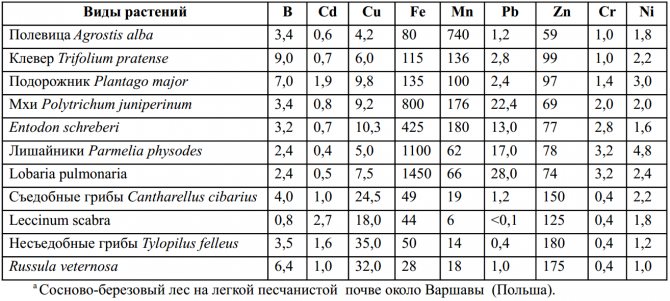

Table 2 - Variations in the content of trace elements in different plant species growing in the same place, in the same forest ecosystema (mg / kg dry weight)
In order to obtain comparable results that could be classified as deficiency, sufficiency, and excess (or plant toxicity), sampling techniques for each field, each crop and specific plant parts in the same development stages should be standardized. Existing soil and plant tests do not adequately predict micronutrient deficiencies for crops, which can lead to errors in micronutrient application.
The concentration ranges of trace elements in mature leaf tissues and their classification, shown in Table 3, are very general and approximate and can vary greatly for particular soil - plant systems. It should be noted that the intervals of trace element concentrations necessary for plants are often close to those concentrations that already have a harmful effect on plant metabolism.Therefore, it is not entirely clear how it is possible to accurately draw the line between sufficient and excessive amounts of trace elements in plants.


Table 3 - Approximate concentration of trace elements in mature leaf tissues according to generalized data for many species (mg / kg dry weight)
Calcium
Normal soil nutrition of plants (mineral) is impossible without calcium, which is present in almost all cells of the plant organism, stabilizing their functionality. This element is especially important for high-quality growth and operation of the root system. Calcium deficiency is accompanied by a delay in root growth and ineffective root formation. There is a lack of calcium in the reddening of the edge of the upper leaves on young shoots. The growing deficit will add a purple color to the entire leaf area. If the calcium does not enter the plant, then the leaves of the shoots of the current year dry up along with the tops.
Toxicity and Tolerance
Metabolic disturbances in plants are caused not only by a lack of microcomponents of nutrition, but also by their excess. In general, plants are more resistant to higher than lower concentrations of elements.
The main reactions associated with the toxic effect of an excess of elements are as follows:
- Change in the permeability of cell membranes - Ag, Au, Br, Cd, Cu, F, Hg, I, Pb, UО2.
- Reactions of thiol groups with cations - Ag, Hg, Pb.
- Competition with vital metabolites - As, Sb, Se, Te, W, F.
- Great affinity for phosphate groups and active sites in ADP and ATP - Al, Be, Sc, Y, Zr, lanthanides and, probably, all heavy metals.
- Substitution of vital ions (mainly macrocations) - Cs, Li, Rb, Se, Sr.
- Capture in molecules of positions occupied by vital functional groups, such as phosphate and nitrate - arsenate, fluoride, borate, bromate, selenate, tellurate, tungstate.
Assessment of toxic concentrations and the effect of trace elements on plants is very difficult because it depends on so many factors that they cannot be compared on a single linear scale. Among the most important factors are the proportions in which ions and their compounds are present in the solution. For example, the toxicity of arsenate and selenate is markedly reduced in the presence of excess phosphate or sulfate, and organometallic compounds can be much more toxic than the cations of the same element, and much less toxic. It should also be noted that some compounds, for example, oxygen anions of elements, can be more toxic than their simple cations.
In the literature, the series of trace elements have been repeatedly cited according to the degree of their toxicity to plants. They are different for each type of experiment and each plant, but they correlate quite well with the following factors:
- electronegativity of divalent ions;
- the product of the solubility of sulfides;
- chelate stability;
- bioavailability.
Despite the discrepancies in the published levels of toxicity, it can be stated that the most toxic for both higher plants and a number of microorganisms are Hg, Cu, Ni, Pb, Co, Cd and, probably, also Ag, Be and Sn.
Although plants quickly adapt to chemical stress, they can still be quite sensitive to excess of a certain trace element. The toxic concentrations of these elements in plant tissues are very difficult to establish. The values given in Table 3 represent a very rough approximation of the probable harmful amounts of trace elements in plants.
The visible symptoms of toxicity vary from species to species and even for individual plants, but the most common and non-specific symptoms of phytotoxicity are chlorotic or brown dots on leaves and their edges and brown, stunted, coral-like roots (Table 7).


Table 7 - The main manifestations of toxicity of trace elements in common agricultural crops
The general property of plants - tolerance - is the ability to maintain vital activity in conditions of an excess of a trace element in the environment, mainly in the soil. Lower plants - microorganisms, mosses, liverworts and lichens - show a particularly high degree of adaptation to toxic concentrations of certain microelements.
Although higher plants are less resistant to elevated concentrations of trace elements, it is known that they can also accumulate these metals and grow in soils contaminated with a large variety of trace elements.
The resistance of plants to the action of heavy metals is of particular importance. Practical challenges and interests regarding metal-tolerant organisms may be related to the following issues:
- microbiological origin of metal ore deposits;
- circulation of metals in the environment;
- geobotanical methods of prospecting for minerals, i.e., the use of tolerant and sensitive plants to search for natural ore deposits;
- microbiological extraction of metals from poor ores;
- growing plants on toxic waste;
- microbiological wastewater treatment;
- development of resistance of microorganisms to metal-containing fungicides and pesticides.
The development of metal tolerance is quite rapid and is known to have a genetic basis. Evolutionary changes caused by heavy metals are now found in a large number of species growing on metal-rich soils. Such changes distinguish these plants from populations of the same species growing on ordinary soils. Higher plant species displaying tolerance to trace elements usually belong to the following families: Caryophyllaceae, Cruciferae, Cyperaceae, Gramineae, Leguminosae, and Chenopodiaceae.
The highest concentrations of trace elements found in various plant species are shown in Table 8. It is known that various fungi are capable of accumulating high concentrations of readily soluble and / or volatile elements such as Hg, Se, Cd, Cu and Zn. The upper critical level of the element is equal to the lowest concentration in tissues at which toxic effects occur. McNichol and Beckett [944] i processed a large number of published data in order to estimate critical levels for 30 elements, of which A1, As, Cd, Cu, Li, Mn, Ni, Se, Zn are the most widely covered. The values of the upper critical levels of concentrations obtained by these authors are quite close to those given in Table 3 in the column "Excess or toxic" concentrations. They also noted that these values for each element are very variable, which reflects, on the one hand, the influence of interaction with other elements, and on the other, an increase in plant resistance to high concentrations of elements in tissues.


Table 8 - The highest accumulation of some metals (% by weight of ash) found in various plant species
The mechanisms of plant resistance to the action of trace elements have been the subject of many detailed studies, which have shown that both highly specific and group tolerance to metals can be observed. These papers summarize the possible mechanisms involved in the creation of metal tolerance. The authors highlight external factors, such as low solubility and low mobility of cations in the environment surrounding plant roots, as well as the antagonistic effect of metal ions. True tolerance, however, is related to internal factors. It does not represent a single mechanism, but includes several metabolic processes:
- selective absorption of ions;
- decreased membrane permeability or other differences in their structure and functions;
- immobilization of ions in roots, leaves and seeds;
- removal of ions from metabolic processes by deposition (formation of reserves) in fixed and / or insoluble forms in various organs and organelles;
- a change in the nature of metabolism - an increase in the action of enzymatic systems that are inhibited, an increase in the content of antagonistic metabolites or restoration of metabolic chains by skipping an inhibited position;
- adaptation to the replacement of a physiological element with a toxic one in the enzyme;
- removal of ions from plants by leaching through leaves, juicing, shedding leaves and excreting through roots.
Some authors provide evidence that tolerant plants can be stimulated in their development by an increased amount of metals, which indicates their physiological need for an excess of certain metals in comparison with the main genotypes or plant species. However, in the physiology of metal tolerance, many points are not yet clear. The resistance of plants to high levels of trace elements and their ability to accumulate extremely high concentrations of trace elements can pose a great danger to human health, since they allow the penetration of contaminants into the food chain.
Magnesium
The process of mineral nutrition of plants during normal development is impossible without magnesium. As part of chlorophyll, it is an indispensable element of the process of photosynthesis.

By activating enzymes involved in metabolism, magnesium stimulates the formation of growth buds, seed germination and other reproductive activity.
Signs of magnesium deficiency are the appearance of a reddish tinge at the base of the leaves, spreading along the central conductor and occupying up to two-thirds of the leaf plate. A strong magnesium deficiency leads to leaf death, a decrease in the productivity of the plant and its decorative effect.
Manganese
Takes part in redox processes and interacts with iron in enzyme systems. With the participation of manganese, which accumulates in the plant, ferrous forms of iron are converted into oxide forms, which eliminates their toxicity. Manganese is involved in the synthesis of vitamins (especially C), enhances the accumulation of sugar in root crops, proteins in cereals. Manganese deficiency is observed on neutral and alkaline soils.
Manganese fertilizers should not be used on soddy-podzolic soils, as well as on strongly acidic soils, on which even the toxic effect of this element on individual crops may appear. However, on carbonate and excessively limed soils, they have a positive effect. Manganese fertilizers are used in the form of manganese superphosphate (2-3%) and manganese sulfate (21-22%).
Boron
Stimulating the synthesis of amino acids, carbohydrates and proteins, boron is present in many enzymes that regulate metabolism. A sign of an acute shortage of boron is the appearance of variegated spots on young stems and a bluish tint of leaves at the base of the shoots. Further deficiency of the element leads to destruction of foliage and death of young growth. Flowering turns out to be weak and unproductive - the fruits are not tied.


We have listed the main chemical elements necessary for normal development, high-quality flowering and fruiting. All of them, correctly balanced, constitute a high-quality mineral nutrition of plants. And the importance of water is also difficult to overestimate, because all substances from the soil come in dissolved form.
Interaction
The balance of the chemical composition of living organisms is the main condition for their normal growth and development. The interaction of chemical elements is of the same importance for plant physiology as the phenomena of deficiency and toxicity. The interaction between chemical elements can be antagonistic or synergistic, and its unbalanced reactions can cause chemical stress in plants.
Antagonism occurs when the joint physiological action of one or more elements is less than the sum of the actions of the elements taken separately, and synergism occurs when the joint action is greater. Such interactions can be associated with the ability of one element to inhibit or stimulate the absorption of other elements by plants (Figure 6). All of these reactions are highly variable. They can occur inside cells, on the surface of membranes, as well as in the environment surrounding plant roots.


1 - antagonism; 2 - synergy; 3 - antagonism and / or synergy; 4 - possible antagonism. Figure 6 - Interaction of trace elements in the plants themselves and in the environment surrounding the plant roots
The interactions between macronutrients and micronutrients, summarized in Table 9, clearly show that Ca, P and Mg are the main antagonistic elements in relation to the absorption and metabolism of many micronutrients. However, even for antagonistic pairs of elements, sometimes synergistic effects were observed, which is probably associated with specific reactions in individual genotypes or plant species.


Table 9 - Interaction between macro- and microelements in plants
Antagonistic effects are most often realized in two ways: the macrocomponent can inhibit the absorption of the microelement, or, conversely, the microelement inhibits the absorption of the macrocomponent. These reactions are observed especially often for phosphates, but were also found for other macrocomponents of nutrition, the consumption and metabolic activity of which were inhibited by a number of microelements.
For practical use, the most important is the antagonistic effect of Ca and P on such heavy metals hazardous to human health as Be, Cd, Pb and Ni.
The interactions between microelements observed in the plants themselves also show how complex these processes are, since they can be either antagonistic or synergistic. Sometimes they manifest themselves in the metabolism of more than two elements (Figure 6). The largest number of antagonistic reactions was observed for Fe, Mn, Cu, and Zn, which are obviously key elements in plant physiology (Table 26). The functions of these trace elements are associated with absorption processes and with enzymatic reactions. Among other trace elements, Cr, Mo and Se are often found in antagonistic relations to this four.
Synergistic interactions between trace elements are usually not observed. The synergism of Cd with trace elements such as Pb, Fe, and Ni may be an artifact resulting from the destruction of physiological barriers by stress caused by excessive concentrations of heavy metals. In addition, some of the reactions occurring in the environment surrounding the roots, and affecting the uptake of trace elements by the roots, do not appear to be directly related to metabolic interactions, however, the two types of reactions are not easily distinguished.
Phosphorus deficiency
With a lack of phosphorus, the leaves become smaller, become dark green, and turn black when dried. The fruits grow sour, their quality is poor. With a lack of phosphorus, signs begin to appear from the lower part of the crown of the tree.
Superphosphate will help eliminate the shortage. But remember to apply fertilizer only at the rate, as much as the tree needs.


Observing garden trees can help you learn about micronutrient deficiencies.
The role of trace elements in plant life
The main role of compounds in the life of green spaces is as follows:
- With a sufficient amount of the latter, the full spectrum of enzymes is synthesized - this allows a greater use of energy and water, to give a greater yield and abundant color.
- These elements help to enhance the regenerating activity of green spaces, preventing their disease.
- It is a sufficient number of them that allows you to strengthen immunity.In their absence, the plant falls into a biological depression and the general susceptibility to parasitic diseases increases.
Trace elements in plant nutrition enhance and accelerate a number of important biochemical reactions.
Trace elements for plants and their role
The biological role of trace elements is great. All plants need microelements to build enzyme systems - biocatalysts. In the absence of these elements, plant life becomes impossible.
The lack of trace elements in the soil does not lead to the death of plants, but is the reason for a decrease in the rate of their development. Ultimately, the plants do not realize their potential and give a low and poor quality yield.
Trace elements for plants are not incorporated into the structure of tissues. In other words, they do not create "body" and "mass". Trace elements function as biological accelerators and regulators of complex biochemical processes. With their deficiency or excess in the soil in vegetables, fruit trees, shrubs and flowers, metabolism is disturbed, and various diseases arise. Therefore, the role of trace elements cannot be underestimated.
Elimination of deficiency or excess of micronutrients
As can be seen from the above material, most of the considered micronutrients have deficiency problems due to inappropriate levels ph... Iron, boron, manganese, copper and zinc - are best absorbed at lower values ph (i.e. in an acidic environment ph <6), while molybdenum, on the contrary, is assimilated at a higher ph (6.5 and even higher).
First:
make sure the level
ph nutrient solution varied smoothly in the optimal range 5,5-6,5. So that every element has a chance to be absorbed by the plant. There is no point in holding ph at some one single and strictly specified mark. It will only bring you problems. And remember ph has a natural tendency to increase, consider this when creating a nutrient solution.
If you understand that the problem is related to ph, rinse the substrate with clean water at a regulated ph, for hydroponic systems - change the solution also to clean water with a regulated ph... This will help restore ph to the appropriate level (required for a particular trace element) and eliminate all nutrient salts that lead to blocking of elements. Start with a clean slate, so to speak.
By the way, the same method works with an excess of any substance!
Second:
often a lack of trace elements occurs when using reverse osmosis or filtered water, when the salt content is close to zero. On the other hand, tap water always contains iron, zinc and other trace elements. Therefore, for those who use osmosis and at the same time got into an unpleasant situation of a deficiency of some element, there is an option to quickly fill the shortage with monofertilizers from
Valagro... To eliminate the deficit molybdenum - Molibion. Zinc replacement - Brexil Zn. Manganese will help restore - Brexil Mn.
Third:
Quite often, micronutrient problems can be a sign of stress. Too dry or hot, underfilling and overflowing, insufficient air circulation inside the greenhouse, insufficient supply of fresh air, little light or, conversely, a lot - there are a million reasons. Check if all the constituent parts of the plant's environment are in order. It often happens that the signs of micronutrient deficiencies will go away on their own with the elimination of stress.
The main thing:
use high-quality fertilizers, the composition of which is balanced and has all the trace elements for plants (preferably in
chelated form). Apply them according to the manufacturer's tables, watch the level ph, and then it is practically guaranteed that problems with deficit (as well as excess) will simply not arise.
Iron (Fe)
The importance of iron for plants
Iron is found in plants in insignificant quantities.The physiological role of iron in plant life is that it is part of enzymes, and also participates in the synthesis of chlorophyll and metabolism. Iron is of great importance in the process of plant respiration, since it is an integral part of respiratory enzymes. Therefore, plant respiration is simply impossible without iron. In addition, since iron is able to pass from an oxidized form to a ferrous form and vice versa, it participates in redox processes in plants.


Iron deficiency - symptoms and how to fix it?
Iron cannot move from old tissues to young ones, therefore, signs of its deficiency appear, first of all, on the upper leaves: they grow immediately completely yellow, and of a bright yellow, almost white color. Iron deficiency leads to the breakdown of growth phytohormones (auxins) synthesized by plants, and therefore plant growth slows down. With an increase in iron deficiency on large leaves, chlorosis appears between the veins, starting from the base of the leaf. In the future, necrosis progresses, and the leaves die off and fall off.
Iron deficiency is usually caused by pH problems. Iron is absorbed best at lower pH values of 5.5-6.0, and at higher pH levels (especially above 7.0) it tends to be blocked. For example, fans of organic growing outdoors should be careful with the use of chicken manure as a fertilizer, since even in small quantities it can greatly increase the pH level of the soil.
True iron deficiency can occur when using filtered or reverse osmosis water to water the plant. When using tap water, the plant receives enough iron, since it is abundant in it.
There are other nutrient problems that cause iron deficiency, such as calcium or magnesium problems, or excess copper can lead to symptoms of iron deficiency. Although iron deficiency sometimes occurs in a stressful environment, it can go away on its own with stress relief.
Excess iron in plants - signs of poisoning
An excess of iron in plants occurs quite rarely, while the growth of the root system and the entire plant stops, the leaves take on a darker shade. If, for some reason, the excess of iron turned out to be very strong, then the leaves begin to die off and crumble without any visible changes. With an excess of iron, it is difficult to assimilate phosphorus and manganese, therefore, signs of a lack of these elements may also appear.
A few rules
Usually, feeding is done in the spring, when the plants start to grow. However, some flowers do not have a pronounced dormant period, while others even bloom in winter. Of course, in this case, they need recharge. But be careful! Keep in mind that the amount of light affects the frequency of fertilization. So, if there is little light, growth and flowering inevitably slows down, nutrients are not used by the roots in full, which means that the earth is salinized. Fast-growing flowers are fertilized once every two weeks, slowly growing once a month, and those that hibernate in winter do not fertilize at all. For the same reason, you should not apply fertilizer on the eve of a dormant period.
When root dressing in dry soil, there is a risk of harming the roots. Pre-moisten with an earthen lump with water, then fertilize.


Microfertilizers: types, application, introduction, properties: video
Microfertilizers: types, application, introduction, properties
TOOL FOR MASTERS AND MASTERS, AND HOUSEHOLD GOODS VERY CHEAP. FREE SHIPPING. RECOMMENDED - CHECKED 100% THERE ARE REVIEWS.
Below are other entries on the topic "How to do it yourself - a householder!"
- DIY wooden flower container - drawing How to make a wooden container for ...
- Solutions for processing and spraying seedlings with your own hands How to prepare solutions for seedlings ...
- Laying a log for the floor - calculation table How to calculate the thickness of the boards and ...
- How to prepare remedies for garden pests with your own hands - folk remedies Infusions and decoctions for pests ...
- How to help trees after: a hurricane, hail, showers and heat: a table-reminder ELEMENTS IN THE GARDEN: ELIMINATE THE CONSEQUENCES ...
- Forest land - harvesting and mixtures with your own hands How to cook leaf land + ...
- How to measure the required amount of fertilizer with the help of improvised means Memo for a gardener - weight ...
Subscribe to updates in our groups and share.
Let's be friends!
With your own hands ›Summer cottage garden and vegetable garden› The introduction of trace elements for plant fertilization - which, when and how much
Calcium deficiency
Calcium in the plant neutralizes excess organic acids. Also, calcium is a potassium antagonism. The correct ratio of calcium and potassium affects the most important life processes in the plant. Calcium deficiency when irrigating with tap water is rare.
Calcium deficiency is manifested:
- The foliage will wither.
- Shoots and foliage turn brown, then die off.
- Excess calcium prevents the absorption of magnesium and potassium.
- Leaves are bent and roots are shortened.
- Frequent fungal infections of the plant.